
.jpg)
For both PSCl 3 and POCl 3, the calculations show that the dissociative electron attachment process is close to thermoneutral. BCl3 Molecular Geometry and Shape Boron forms the central atom in the molecule, with the Chlorine atoms pushing as far. BCl 3, for example, has no dipole moment, while NH 3 does.
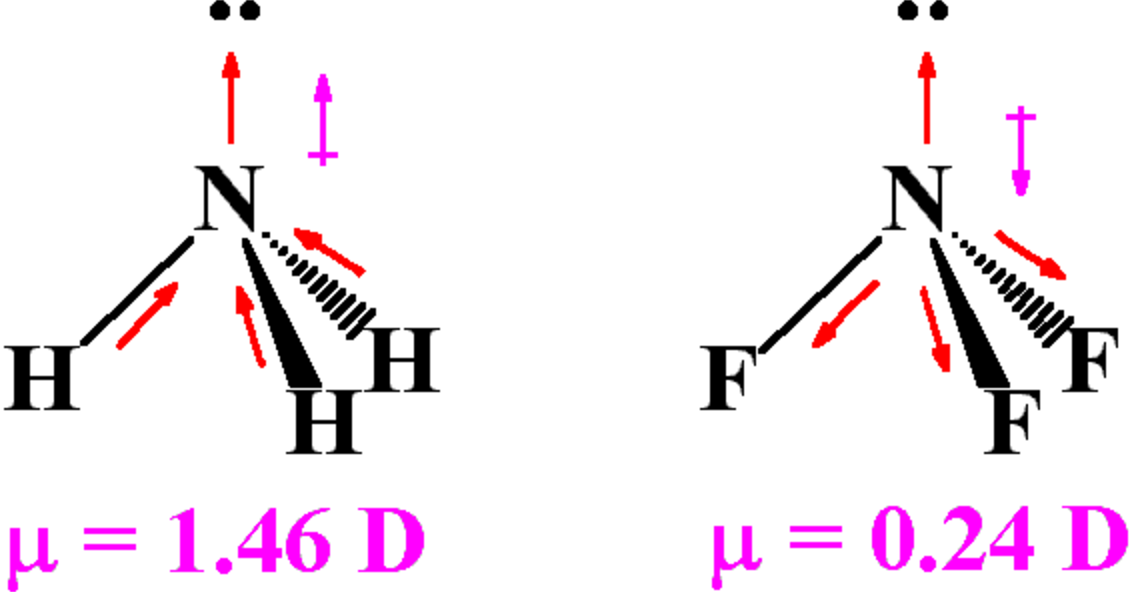
In contrast to PSCl 2, the calculations imply that EA ( POCl 2 ) is slightly greater than EA(Cl). Comparison is made between the present study of electron attachment to PSCl 3 and our earlier work on attachment to POCl 3, and G3 calculations are reported here for neutral and anionic POCl 2 and POCl 3. A natural population analysis was performed to obtain the charges associated with each atom in the molecules in order to estimate how the attached electron is distributed. The calculations indicate that the electron affinity EA ( PSCl 2 ) is slightly smaller than EA(Cl), which may account for the observed branching fractions for PSCl 2 − and Cl − in the low-pressure experiments. Gaussian-3 (G3) calculations were carried out for PSCl 3 and PSCl 2 neutrals and anions to aid in interpretation of the experimental results. In contrast to the low-pressure data, the parent anion product channel ( PSCl 3 − ) was observed (along with the dissociative channels), and increased in importance with N 2 pressure. The three bond-dipoles cancel out, that is, the vector sum of the. This shape gives the molecule a dipole moment. Boron trichloride, BCl3, is trigonal planar and has no net dipole moment, it is nonpolar. Attachment to PSCl 3 was also studied at high pressure (9–93 kPa) of N 2 in an ion mobility mass spectrometer, at 298 K. Which of the following molecules and ions contain polar bonds Which of these molecules and ions have dipole moments a. If B-Cl bond has a dipole moment, explain why BCl 3 molecule has zero dipole moment. The nitrogen atom in the molecule has a lone electron pair, which makes ammonia a base, a proton acceptor. The FALP data suggest that there is an activation energy of about 17 meV for production of PSCl 2 −. Attachment in 133 Pa of He gas yielded only the dissociative ion products PSCl 2 − and Cl −. This rate constant represents an attachment efficiency of about 14%. These experiments yielded an electron attachment rate constant of 5.1×10 −8 cm 3 s −1 that was found not to change significantly in the 298–550 K temperature range. Measurements of rate constants and branching fractions were made in a flowing-afterglow Langmuir-probe (FALP) apparatus. Electron attachment to PSCl 3 was studied in 133-Pa pressure of helium gas at temperatures from 298–550 K. The polarization of the 3 B-Cl bonds exactly cancels out, so BCl3 has no dipole moment.


 0 kommentar(er)
0 kommentar(er)
